A Simple, Powerful Concept.
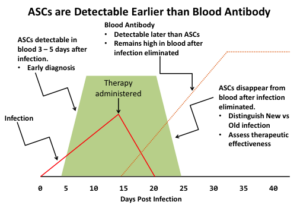
- Earlier results,
- Identifies presence/absence of immune protection,
- Provides indication of therapeutic effectiveness,
- Does not require presence of the pathogenic organism,
- Results obtained with a single whole blood sample and not invasive deep tissue biopsy sample(s),
- Not vulnerable to sampling error (wrong site or contamination by skin flora); and
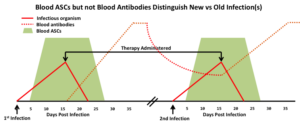
- Not confounded by the patient’s prior immune history.
There are now substantial reports in the scientific literature that support the detection of ASCs for diagnosing infection. These include: Streptococcus pneumonia (lung infection); Respiratory Syncitial Virus (RSV, lung infection); Staphylococcus aureus (orthopaedic infections), Staphylococcus epidermidis (orthopaedic infections); Clostridium difficile (gastrointestinal infection), and Borrelia burgdorferi (Lyme disease). Additionally, federal agencies are investing in MicroBplex’ approach as indicated by federal grants awarded in a highly competitive peer review process. MicroBplex was awarded Phase I and II SBIR grant from the CDC to create a new method for identifying patients at risk for recurrent Clostridium difficile infections (see PRODUCT PIPELINE), and our Scientific Founders have received NIH grants to measure the ASC response to Streptococcus pneumoniae infections (Dr. Lee) and to explore the utility of measuring ASCs for the detection of deep-seated Staphylococcus aureus infections in patients with orthopaedic implants (Dr. Daiss).